ISSN: 0973-7510
E-ISSN: 2581-690X
The aim of this study was to determine the causative agent of acute respiratory infections (ARIs) in hospitalized children, as well as investigate the characteristics of ARIs with single and multiple pathogens detection from July 2015 to June 2016. The objective of the present study was to examine the role of viruses, bacteria and mix infection of both in hospitalized children with ARI and their correlation with two divergent geographical settings of Odisha. Hospitalized children with ARI aged <5 were recruited from July 2015 to June 2016. Nasopharyngeal/Oropharyngeal swabs were collected for detection of common respiratory viruses by reverse transcriptase polymerase chain reaction (RT-PCR).Bacteria were isolated by routine culture methods. The analysis revealed 150 (56%) were detected with >1bacteria, 40(15%) with > 1virus, 22(8.2%) with > 2 bacteria and 20(7-4%) with both bacteria and virus. Most frequently detected pathogens were Klebsiella pneumonae, Setrptococcus pneumonae, Parainfluenza A and Influenza A virus. Incidences of pathogens were detected more among children <1 year, Gender discrimination in the form of dietary neglect of the female children has also been noted mostly in case of tribal patients. Social demographic factors associated with high detection of respiratory pathogens could be responsible for high incidence of respiratory pathogens mostly in tribal population. Till date perhaps no study has been under taken to document the epidemiology of ARI in the state that will facilitate to implement control and prevention measures.
Pathogen, Demographic factor, etiology, Acute Respiratory infection.
The potential threat of acute respiratory infection (ARI) has been recognized as one of the major challenges for its prevention and control in reproductive and child health program1. Currently ARI appears as single largest contributor of under five childhood morbidity and mortality2-4 .A strict definition of ARI would include all infections of the respiratory tract. However, in practice, acute lower respiratory infections accounts for most of the serious disease burden. The major complications of ARI such as pneumonia, acute bronchitis, otitis media and others are thought to cause 4.25 million deaths each year5. ARI causes about 20% of all deaths in pre-school children worldwide, with 90% of these deaths being due to pneumonia. Risk factors for severe ARI include malnutrition, low birth weight, passive smoking, non-breastfeeding, low socio-economic status, overcrowding, immunodeficiency and HIV in developing countries6-8. ARI are caused by viruses or bacteria or due to co-infection of both virus and bacteria.
ARI causing viruses are influenza virus A or B (IFV A/B), Respiratory Syncytial Virus (RSV), Human rhinovirus (RV), Para influenza virus (PIV) and human corona virus. The bacterial pathogens causing ARI include Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumonae, and others. The recent molecular techniques have been employed more frequently for simultaneous detection of multiple pathogens associated with ARI. The report of mix infection of viral and bacterial pathogen is quite common in developing countries that draw critical challenges for medical treatment and clinical management. Besides the association of etiological agents, study of demographic profiles linked with the ARI has significant importance for control of the disease.
It is estimated at least 300 million episode of ARI occur in India every year, out of these about 30 to 60 million are moderate to severe ARI. High morbidity of ARI has been encountered among under- five children admitting or taking treatment in different pediatric hospitals of Odisha.
Odisha an eastern state of Indian situated on the coast of Bay of Bengal and broadly the state is classified into tribal and coastal districts. The state having a total population of about 38 million is the third least urbanized major Indian state, with 15% of population residing in urban areas. It has the highest percentage (47.2%) of population living below poverty line among the other states of India. In comparison to the coastal population, the people in tribal are poor, illiterate and much away from health awareness. High child mortality has been encountered as a major concern in tribal districts due to malnutrition. In general, people of tribal community ignore the health facilities for treatment of children with ARI symptoms. For such a higher proportion of the disease and severity, detail epidemiology should be addressed before implementation of control and prevention measures.
Ethics Statement
The necessary permission for the study was procured from the Chief District Medical Officer (CDMO) of the concerned hospitals and the Institute Ethics Committee (Regional Medical Research Center, ICMR) for research on human beings, and all participants’ guardians gave signed informed consent for participation in the study.
Study population
A hospital based cross sectional study was conducted in Sardarballhab Vai Patel (SVP) Sisubhawan, Cuttack and District Head quarter Hospital (DHH), Rayagada in coastal and tribal region respectively during July 2015 to June 2016. SVP Sisubhawan is a 330 bedded hospital where Pediatric patients visit from different districts for treatment and in case of severe patients, take admission. Similarly DHH in tribal district of Rayagada is a major hospital has 20 bedded pediatric department that caters health services to the pediatric patients coming from nearby and remote areas of the district. Data collection initiated from 2nd week of June 2015 after getting permission from the respective hospitals after preparation of clinical sheet and consent form (English/Oriya) for data collection of the patients.
The study included all hospitalized children with ARI below 5 years of age in two hospitals. Acute respiratory infection in children below five years of age was classified as per the standard case definition [9] that is having one or more of the following symptoms cough, runny-nose, sore throat, chest pain, breathlessness, noise breathing, and fever. The study randomly selected one third of the recruited tribal and costal patients to collect specimen for laboratory testing of ARI aetiology.The purpose of the study was explained to the parent/guardian of all children and informed consent was obtained. Epidemiological information of the cases was collected by interviewing the guardians of the children by using a pre-tested Parforma, information regarding socio-demographic characteristics of the mother and child and associated factors such as overcrowding, cross ventilation, birth order, birth weight, number of siblings, mother’s education, monthly income and smoking habit in the family. Overcrowding was assessed based on the number of persons and living rooms.From recruited patients nasopharyngeal /oropharyngeal swab samples were collected on hospital after obtaining informed consents.
Laboratory detection
Soon after the collection, samples were preserved in virus transport medium (VTM, Himedia, India), transported to the Virology laboratory Bhubaneswar in cold chain and stored at -800C till use.Viral DNA and RNA was extracted from 200µl of VTM medium and eluted in 62 µl AE Buffer by using QIAampMinElute Virus Spin Kits (QIAGEN, Hilden, Germany). The complimentary DNA sample was synthesized by using Super Script First-Strand Synthesis System for reverse transcription polymerase chain reaction (RT-PCR) (Invitrogen, Camarillo, CA). All samples were screened by RT PCR or PCR for detection of common respiratory viruses: influenza virus A or B (IFV A/B), respiratory syncytial viruses (RSV), human rhinovirus (RV), parainfluenza virus (PIV) and human corona virus, etc using standard methods [10]. Bacteriological analyses of the samples were done following the standard microbiological methods [11].Based on colony characteristics and biochemical analysis, different bacteria were identified using standard methods.
Statistical Methods
The data acquired from this study was analyzed and Results on continuous measurements are presented as Mean ± SD, p-value and results on categorical measurements are presented in Number (%).Microsoft word and Excel have been used to generate graphs/tables etc.
Based on the case definition of ARI, 400 children under 5 years of age attending the SVP Sishubhaban, Cuttack and 203 children attending the DHH Rayagada were recruited constituting a total of 603 children. Most of coastal patients73.1% were hospitalized, whereas 46.7% were ambulatory patients in tribal patients (c2 = P< 0.0007). The median age was 1 year (62%) with a range from one day to five years old. Gender difference persisted between patients, females were significantly highly (60.1%) affected in tribal patients where as males were mostly (60.5%) affected among coastal patients. This difference is statistically significant (c2 = P< 0.0001). The most frequent symptoms were fever (67.8%), rhino rhea (61.4%), cough (63.7%), wheezing and respiratory difficulty (54.1%) associated with restlessness and convulsion. Apart from acute respiratory infections the children also had the illness such as vomiting, Dirrhoea, in ability to feed and rashes. Additional demographic and clinical data are presented in Table 1.
Table (1):
Age wise distribution of pneumonia below 5 years age group children
Age |
Broncho Pneumonia (%) |
Pneumonia (%) |
Severe pneumonia (%) |
Any ARI (%) |
Total ARI (%) |
|---|---|---|---|---|---|
0-1 |
243 (72) |
44 (43.5) |
73 (61.9) |
16(34) |
376(62.3) |
1-2 |
46 (13.6) |
24 (23.8) |
28(23.7) |
14(29.8) |
112(18.8) |
2-3 |
18(5.3) |
14(13.8) |
12(10.2) |
5(10.6) |
49(8) |
3-4 |
17(5) |
12(11.8) |
3(2.5) |
7(14.9) |
39(6.7) |
4-5 |
13(3.8) |
7(6.8) |
2(1.7) |
5(10.6) |
27(4.8) |
Total |
337(55.9) |
101(16.8) |
118(19.7) |
47 (7.8) |
603 |
Note.ARI=Acute Respiratory Infection
Majority patients belonged to households with economically backward, at least one smoker, illiterate mother and absence of cross ventilation. On the basis of clinical manifestations the children were associated with Bronchopneumonia 337(55.8%), Pneumonia 101 (16.7%), severe pneumonia 118 (19.5%) and any ARI 47 (7.8%). Among all 376 children of below 1 year age, Bronchopneumonia 243 (64.6%) was identified as one of the predominant disease while severe pneumonia 73 (19.4) was found next to it (Table 2).
Table (2):
Socio-demographic, environmental, and health characteristics of study subjects in coastal and tribal area
| Variable | Category | Number of children (%) | Total (%) | p-value | OR | |
|---|---|---|---|---|---|---|
| Coastal area | Tribal area | |||||
| Socio economic status | U(I) | 46 (11.5) | 15 (7.4) | 61 (10.1) | <0.0001 | |
| U. M (II) | 58 (14.5) | 23 (11.3) | 81 (13.4) | |||
| U. L( III) | 135 (38) | 35 (17.2) | 170 (28.2) | |||
| L (IV) | 161 (40.2) | 130 (64) | 291 (48.2) | |||
| Over crowding | Yes | 249 (62.25) | 149 (73.4) | 398 (66) | 0.0064 | 0.5976 |
| No | 151 (37.75) | 54 (26.6) | 205 (34) | |||
| Gender | Male | 242 (60.5 ) | 81 (40) | 323 (53.6) | <0.0001 | 2.307 |
| Female | 158 (39.5) | 122 (60.1) | 280 (46.4) | |||
| 7Mother’s education | Illiterate | 102 (25.5) | 142 (70) | 244(40.7) | <0.0001 | |
| Primary | 196 (49 ) | 35 (17.2) | 231(38.3) | |||
| Middle/High school | 56 (14 ) | 18 (8.9) | 74 (12.3) | |||
| Higher secondary/Higher | 46 (11.5) | 83 (4) | 129(21.4) | |||
| Number of siblings | 0 | 72 (18) | 17 (8.8) | 89 (14.7) | 0.0002 | |
| 1 | 172 (43) | 74 (36.5) | 246 (40.8) | |||
| 2 | 126 (31.5) | 82 (40.3) | 208 (34.5) | |||
| >2 | 30 (7.5) | 30 (14.8) | 60 (9.9) | |||
| Smoking | Smoking | 148 (37) | 151 (74.38) | 299(49.58) | <0.0001 | 0.2022 |
| No smoking | 252(63) | 52(25.61) | 304(50.41) | |||
| Cross ventilation | Adequate ventilation | 283(70.75) | 163(80) | 446(73.96) | 0.0139 | 0.5936 |
| Inadequate ventilation | 117(29.25) | 40(20) | 157(26) | |||
Note .U=Upper, U.M=Upper Middle, U.L=Upper Lower, L=Lower
The socio-demographic data of the study population in tribal and coastal region were compared to determine the risk factors causing the disease (Table 1). Maximum 64% and 40% of ARI cases were low socioeconomic groups in tribal and coastal region respectively and remaining ARI cases were distributed among low and upper socioeconomic groups (I, II & III) in both the region. Overcrowding was found in 73% families in tribal districts in comparison to the families 63% coastal region. Mother’s illiteracy associated with ARI children was found about 70% and 25% in tribal and coastal regions respectively.
According to the socioeconomic status, the prevalence of ARI was more in low socioeconomic groups compared to other groups in both tribal and costal region. (Class IV, Tribal: 63%, Coastal: 40%, (Table 2). This difference is statistically significant (÷2 = P< 0.001). The comparison of ARI in social classes-IV in coastal verses tribal region the prevalence of ARI was more in tribal (63%) than the coastal population (40%). Overcrowding has the direct influence on the prevalence of ARI: it was higher 62% and 73% in children living in overcrowding houses in both the community of coastal and tribal in comparison to the no overcrowding houses 38% and 27% respectively. Prevalence of ARI was more among male children (60%) in coastal region while more among female children (70%) in tribal region. This association of ARI with male and female children in coastal and tribal population is statistically significant. Prevalence of ARI was more among children having illiterate mother (70%) in comparison to the primary educated mother (17%) in tribal region. In contrast to this result more prevalence of ARI was found among children having primary educated mother (49%) in comparison to the illiterate mother (25%) in coastal region.
Of the total 603 recruited patients, 268 throat swabs samples including 130 from costal and 138 from tribal hospitals could be collected. Bacterial or viral pathogens were detected in 232/268 (86.6%) swab samples. In total samples, there were 150(56%) cases detected with >1 bacteria and 40(15%) cases detected with > 1viruse; 22(8.2%) children detected with > 2 bacteria, no cases detected with > 2 viruses where as 20(7.4%) were co-detected with both viruses and bacteria. Detection of bacteria and virus was more common among children aged <1 year compared to the children of > 1year and incidence was decreasing as age grows (Table 1). On comparison between coastal and tribal area, the detection of bacteria was found more among tribal patients (68%) than the coastal (53.8%).
As a whole the study reveals the detection of bacteria is more 214(79.8%) than the viruses 60(22.4%).The most commonly identified single bacteria were Klebsiella pneumonae 49(18.3%), Streptococcus pneumonae 34(12.7%), E. coli spp 21(7.8%), Staphylococcus aureus 16(6%), Staphylococcus viridians 12(4.8%), Pseudomonas spp 10(1%), and Morexiella spp (3%) (Figure 1). Among the total 214 bacteria identified from among the single and mixed infection, Klebsiella pneumonae 73(34%), Streptococcus pneumonae 48(22.4%), E. coli spp 27(12.6%) and Staphylococcus aurus 27(12.6%) were more frequently detected; Para-Influenza-1 22(36.6%) and Influenza- A 18(30%) viruses were the most frequently detected virus among total 60 viruses. Of the total 22 co-detection of bacteria, Klebsiella pneumonae had higher percentage 19(86.4%) of co-detection with other bacteria (Figure 2). In co-detection of virus and bacteria, Para-Influenza virus contributes higher percentage (50%) of co-detection with other viruses and bacteria (Figure 2). In total 7.5% of children with bacteria detection were co-detected with virus and most frequently co-detected virus were Para-Influenza 1.
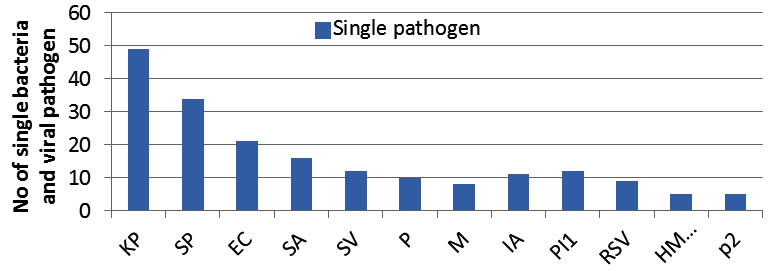
Fig. 1. Detection of single bacteria and virus associated with ARI (Kp, Klebsiella pneumonae;Sp, Streptococcus pneumonia;EC,E.coli;SA,Staphylococcus aureius;SV, Staphylococcus viridian;P, Pseudomonas;M, Morexiella spp;IA, Influenza- A;PI1,Para influenza 1;RSV,respiratory syncytial virus;HMPV,human metapneumovirus ;P2,Para influenza 2 ). Highest number of KP followed by Sp bacterial and PI1 viral pathogens were found associated with ARI.
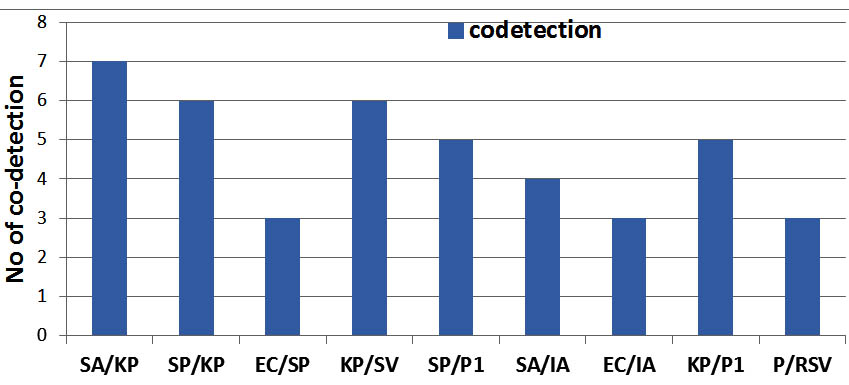
Fig. 2. Co-detection of bacteria-bacteria and bacteria-virus is detected. KP was more frequently detected with SA, SP and PI
Region specific analysis revealed 93 bacteria and 26 viruses in coastal while 121 bacteria and 34 viruses in tribal population. As a whole the comparison of the detection of bacterial and viral pathogens among ARI patients associated with different socio demographic factors demonstrated that higher proportion of bacterial and viral pathogens were detected among the children belonging to households with low socioeconomic group (42.3%), overcrowding (66.4%), illiterate mothers(35.4%), tobacco smoking families(63%) and lack of cross ventilation (59.9%). Again the comparison of the detection of ARI pathogens between the patients belonging to the coastal and tribal community, it revealed that the bacterial and viral pathogen detection was more in tribal than the coastal population among the low socioeconomic group, overcrowding houses, tobacco smoking families, and absence of cross ventilation houses (Figure 3). These results demonstrated that patients belonging to low socioeconomic group, overcrowding, illiterate mothers and lack of cross ventilation were commonly infected by bacterial and viral pathogens.
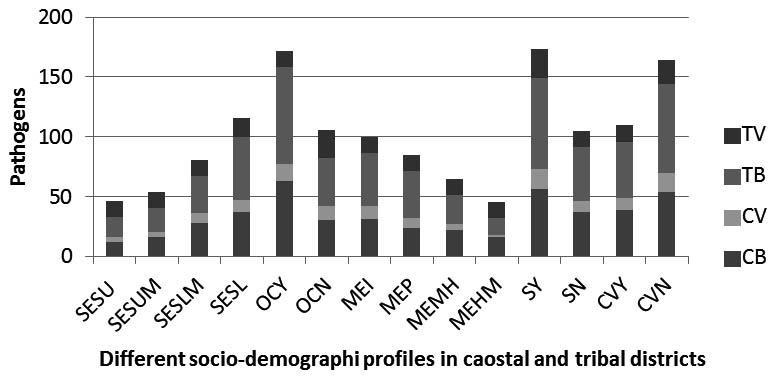
Fig. 3. Diffarent Sociodemographic factors.(SESU, Socio economic status upper class;SESUM, Socio economic status upper middle class;SESLM, Socio economic status upper lower class;SESL, Socio economic status lower class) verses the detection of viruses and bacteria in tribal and coastal(TB,tribal bacteria;TV,tribal virus;CB,coastal bacteria;CV,coastal virus)
This surveillance study demonstrates the association of bacterial and viral pathogens among hospitalized children with ARI. The epidemiology of ARI caused by single and mix pathogens were correlated with different social demographic profiles to address the causative probable social risk factors of infection in two category of population having distracted cultural and social behavior. Different social factors were found to be the core cause of ARI among children in general and more among tribal children in comparison to coastal regions.
To the best of our knowledge, perhaps this is the first report of investigation of ARI among children below five years age associated with single and mixed pathogens of bacteria and virus In Odisha. This study included clinically defined children with fever and acute respiratory infection living in a malaria non-endemic area (coastal) and malaria endemic area (tribal). During the study period 603 under 5 years of age children were hospitalized with fever and among them, 268 swab samples were possible to collect for investigation. To better understand for the role of bacterial and viral agents, we stratified respiratory clinical children into 4 major groups to obtain defined ARI cohorts. The overall positive detection for any respiratory bacteria was 79.8% and 22.4% for virus, with K. pneumonie most commonly detected bacteria and Parainfluenza1, Influenza A, and RSV among viruses. Bacteria were detected more often during illness. The detection of bacteria was significantly greater among samples without viruses compared to samples with viruses. Several previous studies reported the detection of bacterial pathogens in ARI with varied results [12-15]. Among the ARI group, it is well evidenced that viruses are the common cause of common colds. In the virus negative samples, increased detection of bacteria suggests the possibility that bacteria contribute illness pathogenesis. Predominance of viral association for the cause of respiratory infection among children was reported in some etiological studies [16-20], while the present study revealed bacterial predominance for the cause of ARI. In the present study single bacterial detection (54.7%) was more frequent than the viruses (14.6%). Among the single bacterial detection Klebsiella pneumoniae (32.7%) predominantly found followed by Streptococcus pneumoniae (22.7%) and E. coli (14%). Klebsiella pneumoniae shared maximum among the bacteria-bacteria co-detection while Klebsiella pneumoiae and Streptococcus pneumoniae shared equally with Para-1 in co-detection of virus and bacteria. The mechanism of competition or synergism among Streptococcus pneumoniae, H parainfluenza and H. influenza was hypothesized in previous studies ( 16/Wei et al). However in the present study the predominance Klebsiella pneumoiae and Streptococcus pneumoiae suggests their appearance as potential pathogenic organism causing disease keeping behind the other bacteria and viruses in competition. It may also be hypothesized; primary viral invasion might have occurred that in turn facilitated the secondary infection by bacteria particularly more largely by Klebsiella pneumoae.
Among viruses, Influenza-A, Parainfluenza1, RSV, HMPV and Para2 were detected as single pathogen. Some other studies reported the co-detection of viruses with various combinations resulting in interaction which is still unclear about viral etiology and pathogenesis. In contrast to this our study revealed no virus-virus co-detection. In bacteria-virus co-detection, Para-I was found more frequently with Klebsiella pneumoniae and Streptococcus pneumoniae.
Regarding epidemiological analysis of detection of single bacteria and virus, co-detection of bacteria/bacteria and virus/ bacteria, we found that overall detection and co-detection rate was higher among <1 year, where the virus detection is consistent with some other studies on different population [17, 21, 22]. However our higher bacteria detection and co-detection of virus/bacteria among <1 year is contrast to other study that found the mixed viral/bacterial incidence was more common in children aged<2 [23]. However we found that these detection and co-detection rate was more among tribal children compared to the coastal children <1. Our data also indicated that males in coastal district and females in tribal district had higher detection and co-detection of bacteria and virus and this sex difference was described differently in previous literature [22, 24]. Health seeking behavior or sex specific risk might be established for the sex difference which needs further study.
The comparison of pathogen etiology among children belonging to coastal and tribal community demonstrates that the detection of bacteria and virus was more among the tribal children than the coastal. The detection of pathogens among ARI patients when correlated with different socio-demographic factors it revealed low socioeconomic status, overcrowding houses, smoking families and illiterate mother were important risk factors in general but proportionately higher in tribal compared to the coastal districts for transmission of pathogens. However extensive prospective studies are warranted to better understand for the association of bacterial and viral pathogens among ARI patients with different socio-demographic factors as well as geographic regions
ACKNOWLEDGMENTS
The authors are thank full to ICMR, New Delhi and RMRC, Bhubaneswar for funding and providing all the facilities to carry out the research work.SVP Sishubhaban and DHH, Rayagada under health department of government of Odisha is thankfully acknowledged for providing us the facility for data collection and analyze related to ARI. We are also grateful to the Director of the institute for providing us institutional facility.
- Government of India and Ministry of Health and Family Welfare. National Program Implementation Plan RCH Phase II—Program Document, MOHFW, New Delhi, India, 2010.
- Government of India, “Health Status Indicators in National Health Profile”.2009, http://cbhidghs.nic.in.
- WHO, Health Situation in South-East Asia Region 1994-97. Regional office for SEAR, New Delhi, India, 1999.
- United Nations Children’s Fund (UNISEF)/ World Health Organization (WHO). Pneumonia: the forgotten killer. New York, NY, USA, 2006.
- The Acute respiratory Infections Atlas.2010. http//www.ariatlas.sorg/understanding_aris. Accessed September 15.2014.
- Hinman, A. Global progress in infectious disease control. Vaccine 1998; 16: 1116-21.
- Simons E. Environmental and demographic risk factors risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatatr .2003; 143: S118-26.
- Peat, J., Keena, V., Harakeh, Z., Marks, G. Parental smoking and respiratory tract infection in children. Pediatric Respir Rev. 2001; 2: 207-13.
- Government of India, “Health Status Indicators in National Health Profile”.2009, http://cbhidghs.nic.in
- Glezen, W.P., Loda, F.A., Clyde, W.A. Jr, Senior, R.J., Sheaffer, C.I., Conley, W.G., Denny , F.W. Epidemiologic patterns of acute lower respiratory disease of children in pediatric group practice. J Pediatr 1971; 78: 397-06.
- Cook, J.H., Pezzlo, M. Specimen receipt and accessioning. Section 1. Aerobic bacteriology, 1.2.1-4. In HD Isenberg (ed) Clinical Microbiology Procedures Handbook. American Society for Microbiology, Washington DC 1992.
- Heald, A., Auckenthaler, R., Borst, F., Stalder, H. Adult bacterial nasophayngitis: a clinical entity? J Gen Intern Med 1993; 8: 667-73.
- Winther, B., Brofeldt, S., Gronborg, H., Pedersen, M., Vejlsgaard, R. Study of bacteria in the nasal cavity and nasoprynx during naturally acquired common colds. Acta Otolaryngal 1984; 98:315-20.
- Han, J.K., Hendley, J.O., Winther, B. Bacterial pathogens acute sinusitis in the osteomeatal comlex during common colds and wellness. Int forum Allergy Rhinol 2011; 1(5):356-60.
- Allen, E.k., Pitkaranta, A., Maki, M., Hendley, J.O., Laakso, S., Sale, M.M., Winther, B. Bacteria in the nose of young adults during wellness and rhinovirus, colds: detection by culture and microarray methods in 100 nasal lavage specimens. Int Forum Allergy Rhinol.2013; 3(9):731-9.
- Wei, L., Liu, W., Zhang, X.A., Liu, E.M., Wo, Y., Cowling BJ, Cao WC. Detection of viral and bacterial pathogens in hospitalized children with acute respiratory illness, Changing, 2009-2013. J Medicine. 2015; 94: e742.
- Cilla, G., Oñate, E., Perez-Yarza, E.G., Montes, M., Vicente, D., Perez-Trallero, E. Virus in community acquired pneumonia in children aged less than 3years old: high rate of viral co-infection. J. Med Virol 2008; 80: 1843-49.
- Hasan, R., Rhodes, J., Thamthitiwat, S., Olsen, S.J., Prapasiri, P., Naorat, S., Chittaganpitch, M., Henchaichon, S., Dejsirilert, S., Srisaengchai, P., Sawatwong, P., Jorakate, P., Kaewpwan, A., Fry, A.M., Erdman, D., Chuananon, S., Amornintapichet, T., Maloney, S.A., Baggett, H.C. Incidence and etiology of acute lower respiratory tract infection in hospitalized children younger than 5 years in rural Thailand. Pediatr Infect Dis J .2014; 33: E45-E52.
- Khor, C.S., Sam, I.C., Hooi, P.S., Quek, K.F., Chan, Y.F. Epidemiology and seasonality of respiratory viral infection in hospitalized children in Kuala lumpur, Maayasia: a retrospective study of 27 years. BMC Pediatr 2012; 12; 32.
- Falsey, A.R., Becker, K.L., Swinburne, A.J., Nylen, E.S., Formica, M.A., Hennessey, P.A., Criddle, M.M., Peterson DR, Baran A, Walsh EE. Bacterial complication of respiratory tract viral- viral illness: a comprehensive evaluation. J Infect Dis 2013; 208: 432-41.
- Drews, A.L., Atmar, R.L., Glezen, W.P., Baxter, B.D., Piedra, P.A., Greenberg, S.B. Dual respiratory virus infection. Clin Infect Dis 1997; 25:1421-29.
- Chorazy, M.L., Lebeck, M.G., McCarthy, T.A., Richter, S.S., Torner, J.C. Polimicrobial acute respiratory infections in a hospital-based pediatric population. Pediatr Infect Dis J 2013; 32:460-66.
- Korppi, M. Mixed microbial etiology of community acquired pneumonia in children. APMIS 2002; 110: 515-22.
- Nair, H., Simões, E.A., Rudan, I., Gessner, B.D., Azziz-Baumgartner, E., Zhang, J.S., Feikin, D.R., Mackenzie, G.A., Moïsi, J.C., Roca, A., Baggett, H.C., Zaman, S.M., Singleton, R.J., Lucero, M.G., Chandran, A., Gentile, A., Cohen, C., Krishnan, A., Bhutta, Z.A., Arguedas, A., Clara, A.W., Andrade, A.L., Ope, M., Ruvinsky, R.O., Hortal, M., McCracken, J.P., Madhi, S.A., Bruce, N., Qazi, S.A., Morris, S.S., El Arifeen, S., Weber, M.W., Scott, J.A., Brooks, W.A., Breiman, R.F., Campbell, H.; Severe Acute Lower Respiratory Infections Working Group. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 2013; 381:1380-90.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


