The monkeypox virus (MPXV) has become a dangerous zoonosis. The fast spread of MPXV occurred in the last two years. The number of cases remarkably increased in 2022. The reasons behind the sudden increase in MPXV cases are multifactorial. Monkeypox (MPOX) a viral zoonotic illness, is caused by MPXV. It is an enveloped, linear, double-stranded DNA virus. MPXV transmission may take place by direct contact with humans or animals. This article summarizes a better understanding of the spread of MPXV infections. Pregnant and breastfeeding mothers require a high level of care and precaution against this virus as the infection may transmit during pregnancy from mother to fetus and during breastfeeding to the infant. Clinical management of monkeypox in pregnancy is also reviewed in this article.
Monkeypox Virus, Orthopoxvirus, Treatment, Pregnancy
Over the past ten years, cases of new deadly viral diseases have been ongoing.1 The monkeypox outbreak in 2022 affected various countries in both endemic and non-endemic regions.2,3
Monkeypox (MPOX), a rare zoonotic viral illness, is caused by the monkeypox virus (MPXV). MPOX virus belongs to the family Poxviridae, the Orthopoxvirus genus, and the subfamily Chordopoxvirinae. Before the year 2022, MPOX human cases were especially prevalent in Central and West Africa.4,5 The primary MPOXV outbreaks were reported in 1970, 1996–1997, 2003, and 2018. The MPOX disease is milder, with symptoms like those of smallpox, including swollen lymph nodes, high fever, headache, and skin rashes. The skin rashes developed into pus-filled blisters that quickly spread across the entire body, including the face, before they ruptured and crusted after a few days. MPOX is a self-limiting condition that has a 2 to 4-week symptom duration. The mortality rates of MPOX cases range from 1 to 10%.6,7 MPOX cases are reported in both Europe and North America. Since the first case was officially reported in Europe in early 2022, more than 800 suspected or confirmed MPOXV cases are reported in 20 non-African nations.8 The first MPOXV patient was diagnosed in 2022 in the United Kingdom (UK) after travelling from Nigeria; however, some of the recent confirmed cases had no travel history to either Africa or Nigeria, indicating that the Monkeypox virus had started spreading within the community.9 Most cases documented in non-endemic nations are from bisexual, gay, and other males who were involved in sex with males. The MPOX outbreak has afflicted human settlements with no travel connections to an endemic African location, in contrast to the earlier isolated cases.10 Instead of persistent human-to-human transmission, MPOXV penetrates communities in endemic contexts by zoonotic transmission.11,12 There is no confirmation about the reservoir host. Tiny mammals like elephant shrews and rodents may contribute to the natural history of the virus.13 Even though the vaccine used for smallpox shows 85% protection against MPOXV,14 inoculation against the smallpox virus was not offered since 1980,15 when the WHO declared the smallpox virus to be extinct. MPOXV-specific therapeutic agents and immunization are also lacking. A thorough study of the biological traits and pathogenicity of MPOXV is required to stop the spread of MPOX outbreaks. Here, we highlighted the origin, biological characteristics, epidemiology, pathogenicity, signs and symptoms, laboratory diagnosis, prevention, and treatment of MPOX in humans and pregnant women.
History of Monkeypox
Monkeypox is a formerly neglected zoonotic disease indigenous in Central and West Africa. MPOXV was discovered for the first time in 1958 in some captive areas of cynomolgus monkeys. The monkeys were brought from Africa and housed in Copenhagen, Denmark. The virus was like smallpox.16 The first human case of MPOX was reported in the Democratic Republic of the Congo (DRC) in 1970. Tiny forest mammals were found to be the primary carrier of virus transmission and it was later found in different species, like squirrels, rats, prairie dogs, and mice.17,18 In 2003, 47 cases of MPOX outbreak outside Africa were recorded in the six states of the USA.19,20 Some other cases reported outside of Africa were from Israel, Singapore, and the UK. The West African clade and the Central African clade are the two subvariants of the monkeypox virus. The Central African Clade is less aggressive than the West African Clade. The Western African Clade, have an estimated death rate of 1 to 3%, and the Central African Clade has a mortality rate in the range of 10%.18 Although the method of zoonotic transmission of monkeypox to humans is still unknown, the majority of monkeypox infections in humans were spread by contact with small forest animals.16 Following the 2003 US outbreak, a study found that the leading causes of 47 human MPOX cases were brought on by contact with sick animals, bites, scratches, cleaning animal cages, or contact with sick animals’ bedding.19-21 However, some infected people claimed that the presence of infected animals in the same room may cause disease. Similar to the closely related smallpox virus, it is believed that contact with large respiratory droplets and contaminated bodily fluids causes most of the human-to-human transmission.22 The monkeypox virus may also be present in semen and other bodily fluids, according to recent findings.23 However, more research is needed to determine how seminal fluid affects the spread of the monkeypox virus. It is unknown whether pre-symptomatic and asymptomatic carriers exhibit Contagiosity.19 Anecdotal comments from frontline healthcare professionals posted on social media also imply that an infected person may have few or no previous symptoms, which may transmit the virus to another person.24
Biological Characteristics of MPOXV
The MPOX virus particles are 200–250 nm in size and oval or brick-shaped.25 External enveloped virus (EVV) and intracellular mature virus (MV) are the two infectious viral particles produced due to poxvirus replication. The envelope is made up of lipoprotein that surrounds the core of the virus and the lateral body. It contains specific proteins and is present on the surface of the MV. When a cell is ruptured, MV is released which is generally stable in the outer environment. It is mainly spread from the animal-to-animal transmission. A lipid membrane is covered around MVs to create EV, which is released through exocytosis. It comes from endosomes or the transport Golgi apparatus.26 The genome of MPOXV is double-stranded, linear, and around 197 kb in size.27 MPOXV strains encode more than 190 open reading frames (ORFs). The virus’s non-conserved genes are typically found in ITRs at both ends, which are host-and poxvirus-specific. However, four viral proteins (D8, A27, A26, and H3) promote MV adsorption on the surface of cells for the vaccinia virus (VACV), which is most likely MPOXV. MPOXV can last a prolonged period at 4°C and is resistant to drying and cold temperatures.25
Distribution of MPOX
WHO recorded 16,016 cases of monkeypox from 1 January 2022 to 22 July 2022, with laboratory confirmation and five fatalities from 75 countries. DRC provides the number of suspected cases reported starting in 2022 in (Table 1 and Table 2) highest number of monkeypox cases is given.26-28
Table (1):
Human monkeypox cases in the Democratic Republic of the Congo (DRC).
Previous data of 10 Years |
Suspected |
Confirmed |
Death |
|---|---|---|---|
1970 to 1979 |
nil |
48 |
8 |
1980 to 1989 |
nil |
357 |
36 |
1990 to 1999 |
nil |
520 |
3 |
2000 to 2009 |
10027 |
139 |
1 |
2010 to 2019 |
18788 |
283 |
30 |
Table (2):
Highest number of Monkeypox cases in 2022 in five countries.
Country Name |
Number of cases |
|---|---|
Spain |
3125 |
United States of America (USA) |
2316 |
Germany |
2268 |
The United Kingdom (UK) and Northern Ireland |
2137 |
India |
04 |
Sources of infection
Monkeypox is a zoonotic infectious illness that typically manifests intermittently in tropical forests in Central and West Africa.29 MPOXV transmits by both direct touch and vast droplets of inhaled fluid. The vaginal, anal, or oral sex with an MPOX-infected person may spread this virus.30 The Vervet monkeys from Kenya, Chimpanzees, African elephants, wild bears, antelope, Gambian-poached rats, pet prairie dogs, West African squirrels, and anteaters are just a few examples of specific animal reservoirs.31,32
The mode of transmission
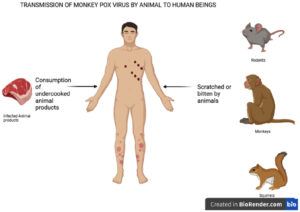
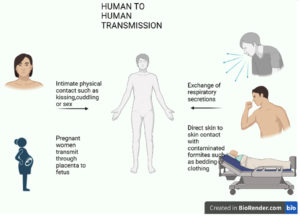
The MPOX virus can spread when an individual meets an infected animal, infected human being, or virus-contaminated items. The transmission of MPOXV is possible from both animal-to-human and human-to-human contact, as shown in Figures 1 and 2, respectively.18 The placenta of a mother can also transmit the virus to the fetus. MPOXV transmission also occurs through direct contact with the blood, other body fluids, or cutaneous or mucosal lesions from bites or scratches of infected animals, treating wild animals, or using items derived from infected animals.33,34 Direct contact with infectious ulcers, scabs, human fluids, or objects such as clothing or linen is the primary method of transmission for monkeypox. Respiratory secretions can also spread during prolonged contact with the face. Additionally, MPOXV can transmit through close physical contact, such as kissing, embracing, or touching monkeypox-infected body parts.
Figure 2. Represents the transmission of the monkeypox virus by infected humans to normal human being.33-35
According to one study, secondary transmission caused up to 28% of cases in the DRC.36 Although the illness is not typically thought of as sexually transmitted, intimate contact has been shown to cause inter-human transmission.37 As a result, the possibility of communal transmission exists. The mode of transmission of MPOXV is shown in Table 3.38
Table (3):
Mode of Transmission of monkeypox virus in recent years.
Year |
Central countries |
Western countries |
Other countries |
|---|---|---|---|
1970 – 1979 |
Cameroon: not known DRC: Both |
Liberia: not known Nigeria: not known Nigeria: human-to-human Sierra Leone: Not known |
nil |
1980 – 1989 |
CAR: Animal to human DRC: Both Gabon: unknown |
Ivory Coast: Not known |
nil |
1990 – 1999 |
DRC: Both Gabon: not known |
nil |
nil |
2000- 2009 |
DRC: Animal to human RC: Animal to human |
nil |
nil |
2010–2019 |
CAR: Both DRC: Animal to human RC: not known |
Nigeria: Both Sierra Leone: not known Sierra Leone: Animal to animal |
Israel: Animal to human Singapore: Animal to human South Sudan: Human-to-Human UK: Human to human US: Animal to human |
Abbreviation: CAR: Central African Republic; RC: Republic of Congo
Monkeypox in pregnancy
The MPOXV can be passed from mother to fetus during pregnancy or from mother to infant during and after birth through intimate contact. MPOX infection during pregnancy can cause unfavourable pregnancy outcomes, such as spontaneous pregnancy loss and stillbirth. Additionally, recorded cases include preterm birth and MPOX infection in newborns. Human monkeypox virus can cause a severe congenital infection and pregnancy loss.39 Table 4 summarised some data on four pregnant women from DRC who got infected with MPOXV during pregnancy.
Table (4):
Observations of conditions of some pregnant women infected with the Human monkeypox virus from DRC in 2007 to 2011.
Pregnant women |
Condition after infection with monkeypox virus |
|---|---|
1st woman |
Spontaneous early miscarriage |
2nd woman |
Spontaneous early miscarriage |
3rd woman |
Second-trimester loss (18 weeks gestation) |
4th woman |
The fetus had generalized skin rashes and monkeypox DNA present in fetal tissue, umbilical cord, and placenta |
Guidelines and Treatment of Monkeypox in Pregnancy
There are no formal guidelines for pregnant women exposed to MPOXV. Pregnant women’s immune systems change during pregnancy therefore, they are more susceptible to exposure to monkeypox virus. Pregnant, recently pregnant, or nursing women should be offered therapy for MPOX. However, considering the lack of data on treatment effectiveness, the treatment choice will depend on the disease’s stage and severity. The choice of whether to treat and monitor a pregnant person as an outpatient or an inpatient should be made individually after the risks and benefits of the treatment have been reviewed with the patient through shared decision-making. WHO has given clinical consideration for monkeypox in pregnant and breastfeeding women. The data regarding the effect of MPOX in pregnant and breastfeeding women are lacking. Pregnant women with MPOX virus infection exhibit similar early signs of fever, headache, lymphadenopathy, malaise, sore throat, cough, and rash, to the non-pregnant women with MPOX virus infection. The MPOX-infected pregnant women should be isolated for 21 days. The women should be monitored for body temperature, blood pressure, and rashes. Ultrasound fetal surveillance should be conducted to check the growth of the fetus. Some outcomes resulting from the review of these pregnant women infected by the monkeypox virus include vertical transmission, maternal morbidity, miscarriage, severe congenital disorder, and no maternal death. Tecovirimat (antiviral drug) and vaccinia immune globulin intravenous (VIGIV) is safe for treating Human Monkeypox infected pregnant women in severe conditions.
Tecovirimat drug is approved by USFDA for pregnant women. Tecovirimat has no embryotoxic or teratogenic effect in animals. It is unknown whether tecovirimat use during pregnancy helps to prevent congenital MPOX. Even though the drug was present in breast milk in animal trials, it is uncertain whether the levels of tecovirimat released into breast milk are enough for treating a breastfeeding child with MPOX. Therefore, breastfeeding children with MPOX should get independent treatment as required. Cidofovir and brincidofovir are potential alternative antiviral medicines for treating MPOX in the general population. However, research on animal reproduction found evidence that they were teratogenic. As a result, women who are in the first trimester of pregnancy should not take these drugs to treat MPOX. It is unknown whether cidofovir and brincidofovir are found in breast milk; therefore, they should not be used by nursing mothers due to the possibility of significant adverse effects on the infant. The first line of treatment in pregnancy should be tecovirimat. In the second or third trimester, cidofovir may be in special cases. However, nephrotoxicity is a significant side effect of cidofovir.
It is unknown whether vaccine immune globulin intravenous (VIGIV) can harm a fetus during pregnancy or impact future fertility because no animal reproduction research is conducted on it. Immunoglobulins, however, are frequently used during pregnancy for a long time without appearing to have any unfavourable consequences on the developing fetus. Therefore, each patient should be examined for the risks and benefits of receiving VIGIV. The excretion of VIGIV in human milk is unknown. Attention should be taken when giving VIGIV to breastfeeding people because many medications secrete in human milk.
Vaccination with ACAM2000 is contraindicated in pregnant or nursing women due to the danger of miscarriage, congenital abnormalities, and vaccinia virus infection in fetuses and new-borns, as well as the availability of a non-replicating viral vaccine. MVA-BN is a third-generation smallpox vaccine that has been approved in the USA (JYNNEOS), Canada (IMVAMUNE®), and Europe (IVANEX®) and is safer as compared to other antivirals because it contains non-replicating viruses and comparatively fewer adverse effects in pregnancy and breastfeeding.39
Diagnosis of monkeypox
A feverish prodrome, the onset of lymphadenopathy, a generalized rash with well-circumscribed lesions at the same stage of development, and a pattern of centrifugal dispersion are all characteristics of typical MPOX (as shown in Table 5). The recommended diagnostic samples are collected by vigorously swabbing skin, fluid, or crusts directly from the rash. Testing can be done on oropharyngeal, anal, or rectal swabs in the absence of cutaneous lesions. Blood testing is not advised. The time of incubation for MPOX is 3–17 days. A person may feel fine and not have any symptoms during this time. Usually, the disease lasts two to four weeks.40-41
Table (5):
Diagnosis of MPOXV.40,41
Diagnosis tests |
Description |
|---|---|
|
Detects the presence of MPOXV and its specific DNA signatures |
2) Phenotypic Methods (By clinical diagnosis) |
Live virus is taken from the specimen of the patient and grown and characterized |
3) Electron Microscopy |
A brick-shaped particle confirms the presence of pox virus |
4) Immunological methods (ELISA) |
Serological detection of MPOXV through peptide-based ELISA |
a) Anti-OPXV IgG |
Test to detect the presence of orthopox virus (OPXV) specific antibody |
b) Anti-OPXV IgM |
Test to detect the presence of OPXV antibodies |
5) Virus-Host Interaction (Tissue tropism) |
Test to detect the presence of OPXV antibody |
Prevention and Treatment of Monkeypox
MPOXV spread can be prevented by adopting infection prevention and control approaches. Immunization is one such approach. Persons involved in sex with multiple partners or queer sex are prone to get infected. A person infected with MPOXV should be isolated to prevent the spread of the virus to healthy persons. The MPOXV has been found in semen however, the spread of MPOXV through sex is not confirmed. However, direct contact like touching, kissing, or sexual intercourse should be avoided with infected persons.42 The treatment of MPOX includes vaccines and antivirals, as shown in Table 6.
Table (6):
Treatment of Human monkeypox by Vaccines and antiviral drugs.
| Vaccines | Name | Features | Anti pox Virus |
|---|---|---|---|
| Second Generation | ACAM2000™ | Second generation vaccine | MPOXV, Smallpox virus |
| Third Generation | IMVAMUNE | Third generation vaccine | MPOXV Smallpox virus |
| Antiviral drugs | Tecovirimat (ST-246) | Small molecule virus inhibitor | Cowpox virus, MPOXV Smallpox virus |
| Brincidofovir (CMX001) | Viral DNA polymerase inhibitors | MPOXV | |
| Ribavirin and Tiazofurin | IMA dehydrogenase inhibitors | All poxviruses | |
| C-CA3-ADO, C3-NPC A | S adenosylhomocysteine hydrolase inhibitors | All poxviruses | |
| HPMA, Adenosine N1 oxide (ANO) | DNA polymerase inhibitor | All poxviruses | |
| Nioch-14 | Nucleoside analogues inhibitor | MPOXV and vaccinia virus |
Vaccination
There is not yet a specific vaccine available to prevent MPOXV infection. According to certain reports, vaccinating against smallpox offers 85% protection from MPOXV.42,43. IMVAMUNE® (Canada), IVANEX® (Europe) or JYNNEOS (USA) is a replication-deficient, attenuated, third-generation modified vaccinia Ankara Bavarian Nordic (MVA-BN) vaccine that has also received approval from the European Medicine Agency (EMA) and the Food and Drug Administration (FDA) for the prevention of MPOXV and smallpox virus in adults 18 years of age and older in high-risk populations. IMVAMUNE, in contrast to ACAM 2000, can be utilized by people with immunodeficiency disorders and atopic dermatitis44. MVA-BN, LC-16, or ACAM 2000 are the vaccines available for MPOX. However, only MVA and LC-16 are approved against MPOXV.42-45
Antiviral drugs
There are currently no antiviral medications specifically designed to cure MPOX, and interventions are primarily symptomatic and supportive. Drugs that fight the smallpox virus can be effective against MPOXV. Small molecule virus inhibitor tecovirimat (ST-246) possesses potent anti-orthopoxvirus efficacy against the smallpox virus, MPOXV, and cowpox virus. By reducing the primary envelope protein’s (F13L) ability to function, it can stop the propagation of the virus by preventing it from exiting an infected cell.46 For the treatment of MPOX, it received approval in Europe in 2022.47 Cidofovir and the Brincidofovir derivative (CMX001) work as viral DNA polymerase inhibitors. Cidofovir is an acyclic nucleoside phosphonate. The drug’s lipid wrap breaks when CMX001 enters the host cells, releasing free Cidofovir that will then be phosphorylated into Cidofovir-diphosphate (CDV-PP). As a substitute matrix, CDV-PP prevents the production of viral DNA polymerase and finally prevents viral DNA synthesis at the DNA polymerase level.48 In vitro and in vivo MPOXV replication inhibition is possible with Cidofovir and Brincidofovir.48,49 Nioch-14 has potential as an anti-MPOXV medication because it is simple to make.50 IMP inhibitors ribavirin and tiazofurin inhibit all poxviruses. Albeit variola virus (VARV) and MPOXV are more susceptible to their effects 49. The DNA polymerase inhibitors HPMA, adenosine N1 oxide (ANO), and S-adenosylhomocysteine (SAH) hydrolase inhibitors C-CA3-ADO and C3-NPC all demonstrated intense anti-poxvirus activity and may employ as possible anti-MPOXV medications.51-53
It suggests the prospect of long-term undetected transmission followed by recent amplification episodes, given the surprising spread of monkeypox across several non-endemic countries. Fortunately, some COVID-19 prevention techniques, including social disengagement, mask-wearing, surface sanitization, and hand washing, may also effectively stop the transmission of monkeypox. Due to vertical virus transmission from mother to fetus, monkeypox infection during pregnancy can increase the chance of prenatal loss. Preliminary findings indicate the necessity for broad maternal and fetal monitoring during pregnancies. A significant global registry of monkeypox infections in expecting women must be created to assess the true impact of this illness during pregnancy. International cooperation is crucial to containing the MPOX spread and lowering the threat posed by MPOX.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Rajak H, Jain DK, Singh A, Sharma AK, Dixit A. Ebola virus disease: past, present and future. Asian Pac J Trop Biomed. 2015; 5(5):337-43.
Crossref - Media centre. European Centre for Disease Prevention and Control. Published February 12, 2021. Accessed May 24, 2023. https://www.ecdc.europa.eu/en/news-events/
- World Health Organization. Multi-country monkeypox outbreak: situation update. https://www.who.int/emergencies/disease-outbreak-news/ item/2022-DON392
- Magnus PV, Andersen EK, Petersen KB, et al. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959;46(2):156-176.
Crossref - Rizk JG, Lippi G, Henry BM, Forthal DN, Rizk Y. Correction to: Prevention and treatment of Monkeypox. Drugs. 2022;82(12):1343.
Crossref - Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):1153-1162.
Crossref - Ogoina D, Izibewule JH, Ogunleye A, et al. The 2017 human monkeypox outbreak in Nigeria-report of outbreak experience and response in the niger delta university teaching hospital, Bayelsa state, Nigeria. PLoS One. 2019;14(4):e0214229.
Crossref - Kozlov M. Monkeypox outbreaks: 4 key questions researchers have. Nature. 2022;606(7913):238-239.
Crossref - De Baetselier I, Van Dijck C, Kenyon C, et al. Retrospective detection of asymptomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. Nat Med. 2022;28(11):2288-2292.
Crossref - Yadav R, Jha M, Prasad S, Jat D, Jain DK. Mayaro virus (MAYV) Disease: Past, present, and future. J Pharm Biol Sci. 2022; 10(1):7-16.
Crossref - Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872-879.
Crossref - Farahat RA, Abdelaal A, Shah J, et al. Monkeypox outbreaks during COVID-19 pandemic: are we looking at an independent phenomenon or an overlapping pandemic? Ann Clin Microbiol Antimicrob. 2022;21(1):26.
Crossref - Doty JB, Malekani JM, Kalemba LN et al. Assessing monkeypox virus prevalence in small mammals at the human-animal interface in the Democratic Republic of the Congo. Viruses. 2017;9(10):238.
Crossref - Fine PE, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17(3):643-650.
Crossref - Jezek Z, Khodakevich LN, Wickett JF. Smallpox and its post-eradication surveillance. Bull World Health Organ. 1987;65(4):425-434.
- Heymann DL, Szczeniowski M, Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54(3):693-702.
Crossref - Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33(4):1027-1043.
Crossref - Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12(11):1257-1258.
Crossref - Centers for Disease Control (May 20, 2022). Monkeypox. https://www.cdc.gov/poxvirus/monkeypox/index.html
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194(6):773-780.
Crossref - Reynolds MG, Davidson WB, Curns AT, et al. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg Infect Dis. 2007; 13(9):1332-1339.
Crossref - Mahase E. Monkeypox: what do we know about the outbreaks in Europe and North America. BMJ. 2022;377:o1274-o1275.
Crossref - Antinori A, Mazzotta V, Vita S, et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022;27(22):2200421-2200422.
Crossref - Otu A, Ebenso B, Walley J, Barcelo JM, Ochu CL. Global human monkeypox outbreak: atypical presentation demanding urgent public health action. Lancet Microbe. 2022;3(8):E554-E555.
Crossref - Cho CT, Wenner HA. Monkeypox virus. Bacteriol Rev. 1973; 37(1):1-8.
Crossref - Pickup DJ. Extracellular virions: the advance guard of poxvirus infections. PLoS Pathog. 2015;11(7):e1004904.
Crossref - Kugelman JR, Johnston SC, Mulembakani PM, et al. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg Infect Dis. 2014;20(2):232-239.
Crossref - World Health Organization. Africa. WHO is supporting African countries to strengthen monkeypox surveillance and response actions. 2022. Available at: https://www.afro.who.int/news/whosupporting-african-countries-strengthen-monkeypox-surveillance-and-response-actions
- Reynolds MG, Doty JB, McCollum AM, Olson VA, Nakazawa Y. Monkeypox re-emergence in Africa: a call to expand the concept and practice of one health. Expert Rev Anti Infect Ther. 2019;17(2):129-139.
Crossref - Durski KN, McCollum AM, Nakazawa Y, et al. Emergence of monkeypox – West and Central Africa, 1970-2017. MMWR Morb Mortal Wkly Rep. 2018;67(10):306-310.
Crossref - Nolen LD, Osadebe L, Katomba J, et al. Introduction of monkeypox into a Community and household: risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am J Trop Med Hyg. 2015;93(2):410-415.
Crossref - Centers for Disease Control and Prevention. Multistate outbreak of monkeypox–Illinois, Indiana, and Wisconsin. MMWR Morb Mortal Wkly Rep. 2003;52(23):537-540. Pmid: 12803191.
- Van Saene HK, Stoutenbeek CC, Stoller JK. Selective decontamination of the diges-tive tract in the intensive care unit: current status and future prospects. Crit Care Med. 1992;20(5):691-703.
Crossref - Ihekweazu C, Yinka-Ogunleye A, Lule S, Ibrahim A. Importance of epidemiological research of monkeypox: is incidence increasing. Expert Rev Anti Infect Ther. 2020;18(5):389-392.
Crossref - Cuerel A, Favre G, Vouga M, Pomar L. Monkeypox and pregnancy: Latest updates. Viruses. 2022;14(11):2520.
Crossref - Shchelkunov SN, Totmenin AV, Babkin IV, et al., Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509(1):66-70.
Crossref - Vivancos R, Anderson C, Blomquist P, et al. Com-munity transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill. 2022;27(22):2200422.
Crossref - Breman JG, Kalisa-Ruti, Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970-79. Bull World Health Organ. 1980;58(2):165-182.
- D’antonio F, Pagani G, Buca D, Khalil F. Monkeypox infection in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2022;5(1):100747.
Crossref - Altindis M, Puca E, Shapo L. Diagnosis of monkeypox virus-An overview. Travel Med Infect Dis. 2022;50:102459.
Crossref - Nakhaie M, Arefinia N, Charostad J, Bashash D, Haji Abdolvahab M, Zarei M. Monkeypox virus diagnosis and laboratory testing. Rev Med Virol. 2023;33(1):e2404.
Crossref - Brown E, Senkevich TG, Moss B. Vaccinia virus F9 virion membrane protein is required for entry but not virus assembly, in contrast to the related L1 protein. J Virol. 2006;80(19):9455-9464.
Crossref - Nasir IA, Dangana A, Ojeamiren I, Emeribe AU. Reminiscing the recent incidence of monkeypox in Nigeria: Its ecologic-epidemiology and literature review. Port Harcourt Med J. 2018;12(1):1-9.
Crossref - Petersen BW, Kabamba J, McCollum AM, et al. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antiviral Res. 2019;162:171-177.
Crossref - Doshi RH, Guagliardo SAJ, Doty JB, et al. Epidemiologic and ecologic investigations of Monkeypox, likouala department, Republic of the Congo, 2017. Emerg Infect Dis. 2019;25(2):281-289.
Crossref - Yang G, Pevear DC, Davies MH, et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J Virol. 2005;79(20):13139-13149.
Crossref - Thakur V, Thakur P, Srivastava S, Kumar P. Monkeypox virus (MPOX) in humans a concern: Trespassing the global boundaries – Correspondence. Int J Surg. 2022;104(106703):106703.
Crossref - Magee WC, Hostetler KY, Evans DH. Mechanism of inhibition of vaccinia virus DNA polymerase by cidofovir diphosphate. Antimicrob Agents Chemother. 2005;49(8):3153-3162.
Crossref - Delaune D, Iseni F. Drug development against smallpox: Present and future. Antimicrob Agents Chemother. 2020;64(4):01683-19.
Crossref - Baker RO, Bray M, Huggins JW. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 2003;57(1-2):13-23.
Crossref - Joshi P, Loshali A, Ale Y, Parveen G, Saha P, Jakhmola V. Human-to-Human Transmission of Monkeypox Virus Old Virus with a New Face. J Pure Appl Microbiol. 2022;16(suppl 1):3048-3061.
Crossref - Rana S, Negi P, Devi M, Butola M, Ansori ANM, Jakhmola V. Systematic Review On New Face of Monkeypox Virus. J Pure Appl Microbiol. 2022;16(suppl 1):3119-3129.
Crossref - Nainwal N, Jakhmola V. candidate vaccines and therapeutic against monkey pox infection. J Pure Appl Microbiol. 2022;16(suppl 1):3096-3105.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.